A wonderful article (reprinted below) and short video was published last weekend in Phillyburbs.com recognizing the work of Joan Block, the Executive Director and co-founder of the Hepatitis B Foundation. In commemoration of World Hepatitis Day, Joan and Dr. Anna Lok were honored by the Viral Hepatitis Action Coalition of the Centers for Disease Control Foundation, for advocacy work resulting in the protection of medical students from HBV discrimination, and ultimately having HBV recognized as a disability protected under the Americans with Disabilities Act (ADA). This amazing accomplishment is just one of the many successes Joan and the HBF have had over the last 23 years as a result of her tireless efforts and dedication to the mission to help improve the lives of those affected by hepatitis B.
Please visit Phillyburbs.com to access to view the short video where Joan talks about the Foundation’s beginnings and how the HBF has grown from a grass roots effort to the leading national nonprofit for hepatitis B. Joan Block and the HBF truly are the “voice of hepatitis B”.
Read more about the story and the mission of the Hepatitis B Foundation and be sure to visit the HBF website to learn more about hepatitis B and the work of the Foundation.
For more than two decades, the Hepatitis B Foundation has fought to find a cure for the liver disease and advocate for those who have it.
What started as a grass-roots effort of four passionate people has grown into one of the leading nonprofit research and disease advocacy organizations in the United States.
“We are the voice for hepatitis B in the United States,” said co-founder and executive director Joan Block. “There’s still a lot of work to do, but we’ve accomplished a lot in the past 23 years.”
Earlier this year, the foundation’s mission got a boost when the U.S. Department of Justice said hepatitis B patients are protected under federal disability law in a case brought by the foundation against a New Jersey medical school on behalf of two students who were denied admission because they had the disease.
The case earned Block and Dr. Anna Lok, director of clinical hepatology at the University of Michigan Health System, recognition from the Centers for Disease Control Foundation, which honored both women on World Hepatitis Day July 25.
“That award is really being given to the foundation,” said Block, who lives in Doylestown Township. “It’s not me; it’s the work of the foundation. Without the foundation, I honestly don’t know if hepatitis B would even have much on the radar screen. There are very few voices, and we are probably the primary voice at the national level.”
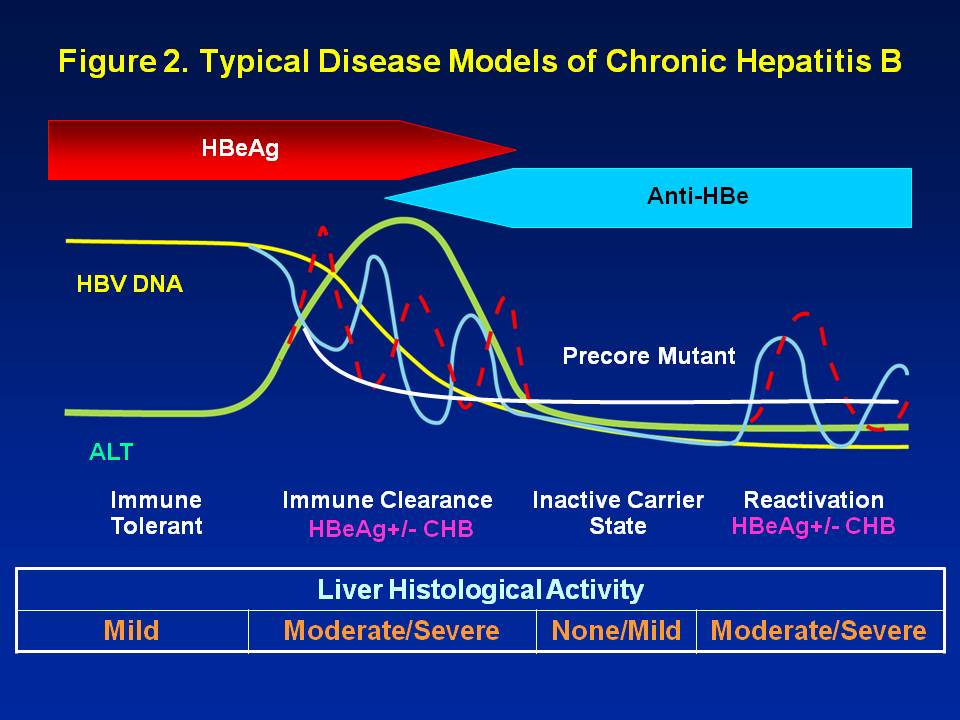
Hepatitis B is an infectious liver disease that can be spread by sexual contact, sharing infected needles or at birth from mother to child, according to the Centers for Disease Control and Prevention. Chronic infection can lead to liver failure and cancer.
Block, her husband, Timothy Block — a researcher and academic who more often serves as the public face of the foundation — and New Hope philanthropists Jan and Paul Witte founded the Hepatitis B Foundation 23 years ago to draw more attention to and develop a cure for the disease, which affects up to 1.4 million Americans.
The couples initially started out to help a local family dealing with the disease. But what they found was little interest from the public health sector in researching a cure. The hepatitis B vaccine has led to dramatically lower rates of infection, and the prevailing, yet incorrect, belief at the time was that the disease infected mainly gay men and intravenous drug users.
“The more we dug, the more we realized there was nothing out there,” Joan Block said. “It was really grass roots, just the four of us in (the Wittes’) kitchen. We had the grand mission of raising a lot of money to start a research effort. That’s really what we needed. But it was hard to raise money when people didn’t know what hepatitis B was.”
Over the years, she said, the foundation has received numerous phone calls from people who believed they were being discriminated against because they have the disease. Some of them were medical professionals.
In 2011, four students contacted the foundation over six months, all claiming they were denied admission to or kicked out of medical and dental schools after discovering they had hepatitis B. All four were Asian Americans who were infected at birth. About half of infected patients in the U.S. are of Asian descent.
“It seemed like an avalanche,” said Block, a registered nurse who taught at Abington’s nursing school.
The foundation and Lok lobbied the CDC to update hepatitis B guidelines for medical professionals and students last changed in 1991. Since that last update, there have been no reports of hepatitis B transmission from medical or dental students, according to the CDC.
The Hepatitis B Foundation also filed a lawsuit on behalf of two students denied admission to the University of Medicine and Dentistry of New Jersey. The school settled with the Department of Justice.
In an added step, the departments of justice, health and human services and education sent a joint letter to the nation’s medical and dental schools about hepatitis B, encouraging them to adopt the CDC guidelines and informing them that people with hepatitis B are protected under the Americans with Disabilities Act.
But the foundation’s advocacy work is far from over. The organization is now lobbying on behalf of servicemen and women who are fighting discharge from the military on the grounds that they’re infected with the disease.
And the foundation’s executive director continues to push for a cure.
“We still want that cure,” Joan Block said. “We’re not satisfied that it’s preventable and controllable. We still have an urgent mission. That has not changed.”