- TBK1 and IKKε Act Redundantly to Mediate STING Induced NF-kB Responses in Myeloid Cells – Cell Reports
- This paper from The Walter and Eliza Hall Institute of Medical Research in Melbourne, Australia deciphers the role of the kinases TBK1 and IKKε in STING-induced, NF-kB-mediated cytokine production. Stimulator of Interferon Genes (STING) protein is a vital component of the innate immune system. Cyclic guanosine monophosphate-adenosine monophosphate (cGAMP) synthase (cGAS), is a pattern recognition receptor (PRR) that senses cytoplasmic double-stranded DNA (dsDNA). In response to dsDNA binding, cGAS catalyzes the production of 2’3′-cGAMP, a cyclic dinucleotide (CDN) which activates STING by direct binding. Once bound to 2’3′-cGAMP, STING dimers undergo a conformational change and translocate from the endoplasmic reticulum (ER) to the Golgi apparatus. At the Golgi, the serine-threonine protein kinase TANK-binding kinase 1 (TBK1) phosphorylates STING at residues in its C-terminal tail (CTT). This phosphorylation causes the recruitment of interferon regulatory factor 3 (IRF3) to STING which is also phosphorylated by TBK1. Phosphorylated IRF3 forms dimers and translocates to the nucleus where it induces the expression of type I interferons (IFN-I) such as IFN-β. IFN-I production and secretion lead to the activation of numerous IFN-stimulated genes (ISGs) which induce a robust antiviral state in the cell. Concomitant to IFN-I induction, STING activation is also known to induce a set of proinflammatory cytokines through the transcription factor called nuclear factor-kB (NF-kB). These cytokines include tumor necrosis factor alpha (TNFα) and interleukins (IL) IL-1β and IL-6. While TBK1 and to a much lesser extent IkB kinase ε (IKKε) are needed for IRF3-mediated IFN-I transcription, several lines of evidence indicate that they may be unnecessary for STING-induced NF-kB activity. For instance, the CTT region of STING, critical to IFN induction, is observed only in vertebrates. While STING activation in the invertebrate species Drosophila melanogaster and Nematostella vectensis results in NF-kB-mediated transcription of cytokines, it does not induce IFN-I transcription. Additionally, ubiquitination of STING at lysine residues K244 and K288 which is required for its trafficking from the ER to the Golgi is essential for IFN-I induction, but not for NF-kB activation. Finally, phosphorylation of STING at serine residues S358 and S366 in the CTT is required for IRF3 activation but is unnecessary for NF-kB activity. This publication reports that while TBK1 kinase activity is critical for IRF3 activation, TBK1 and IKKε act redundantly and in a kinase-independent manner to activate NF-kB signaling. To determine this, conditional TBK1-knockout mice were generated. These mice were the offspring of mice “floxed” for TBK1 and “RosaCre” mice (ROSA26-CreERT2). The floxed mice were mutated to have their TBK1 gene sandwiched between two lox P sites (Tbk1fl/fl). The RosaCre mice were mutated to constituatively produce a fusion protein of the Cre recombinase and the estrogen receptor (CreER). The TBK1 conditional knockout mice (Tbk1fl/fl x RosaCre) transcribe TBK1 until they are treated with the synthetic steroid tamoxifen. Tamoxifen binds the the CreER fusion protein (CreERT) and causes its translocation to the nucleus where it binds to lox P sites and its recombinase activity causes the deletion of the TBK1 gene. Conditional knockout mice had to be used to study TBK1 because complete constituative TBK1 knockout is lethal to mice. Primary bone marrow-derived macrophages (BMDM) were obtained from both tamoxifen-treated wild-type Tbk1fl/fl (WT) and Tbk1fl/fl x RosaCre (TBK1 knockout) mice. When subjected to the STING agonist 2’3′-cGAMP, BMDMs from WT mice showed phosphorylation of IRF3 by Western blot and secretion of IFN-β by ELISA. Under the same treatment, BMDMs derived from TBK1 knockout mice showed drastically reduced IRF3 phosphorylation and IFN-β secretion. Interestingly, BMDMs derived from both WT and TBK1 knockout mice secreted similar levels of TNFα when treated with 2’3′-cGAMP. Next, BMDCs from normal mice were immortalized and CRISPR/Cas9 was used to knockout expression of TBK1, IKKε, or both. Significantly, while TNFα secretion upon 2’3′-cGAMP treatment was modestly reduced by the knockout of either TBK1 or IKKε, it was almost completely ablated by the knockout of both genes. Interestingly, knockout of both genes had no effect on the secretion of TNFα in response to treatment with lipopolysaccharide (LPS). Finally, in order to determine the upstream signaling responsible for STING-mediated NF-kB activity, two proteins were investigated: transforming growth factor b-activated kinase 1 (TAK1) and inhibitor of nuclear factor kappa-B kinase subunit beta (IKKβ). Small molecule inhibitors were used to inhibit TAK1 and IKKβ prior to treatment with the mouse STING agonist DMXAA. Inhibition of both TAK1 and IKKβ resulted in diminished NF-kB activity, implicating their role as kinase activators of NF-kB downstream of STING. Taken together, these results indicate that TBK1 and IKKε act redundantly to carry out STING-mediated NF-kB activity. Additionally, it is likely that TAK1 acts downstream of TBK1 and IKKε to activate the IKK complex, resulting in NF-kB activity. This finding has direct therapeutic significance for STING-driven autoimmune disorders such as chronic polyarthritis. Many strategies for overcoming such diseases only target the IFN-I-producing pathway, while pro-inflammatory cytokine production may go unchecked. This finding elucidates a less-studied arm of STING signaling which is important for basic science and future therapies.
- Glycoengineered Hepatitis B Virus-Like Particles with Enhanced Immunogenicity – Vaccine
- This paper from the Royal Melbourne Institute of Technology University in Melbourne, Australia shows that an HBV vaccine using glycosylated HBV surface protein may have better efficacy than the current vaccine. HBV encodes three surface proteins (large, medium, and small) which are truncated forms of the same protein. The small HBV surface protein (HBsAgS) contains the major antigenic determinants of the protein. In the absence of other viral proteins, HBsAgS will self-assemble into non-infectious particles termed subviral particles (SVP), also known as virus-like particles (VLP). VLPs are the major species of HBV viral particle secreted from infected hepatocytes. When grown in mammalian cells in vivo, approximately half of HBsAgS molecules receive N-glycosylation at asparagine residue N146. N-glycosylation is the addition of an oligosacharide molecule to the nitrogen atom of an asparagine residue within a protein. These modifications occur in the endoplasmic reticulum (ER) and are important for the function of proteins and for signaling within the cell. The current HBV vaccines are composed of HBsAgS VLPs grown in yeast. In contrast to VLPs grown in mammalian cells, yeast-derived VLPs have no N-glycosylation. Additionally, HBV vaccines contain adjuvants which aid in immune system stimulation. The widely-used HBV vaccines Engerix-B (GlaxoSmithKine) and Recombivax HB (Merck) contain the adjuvants aluminum hydroxide and aluminum hydroxyphosphate respectively. Aluminum salts stimulate the immune system by causing activation of the NLR family pyrin domain-containing protein 3 (NLRP3) inflammasome pathway. Upon vaccination, aluminum salt crystals are taken into local dendritic cells via phagocytosis where they rupture the lysosome, causing activation of the NLRP3 inflammasome which includes active caspase 1. The catalytic activity of caspase 1 cleaves pro-interleukin 1β (IL-1β) as well as gasdermin D into their active forms. Cleaved gasdermin D forms pores in the cell membrane resulting in the rapid release of pro-inflammatory IL-1β and ultimately causing pyroptosis, an immunogenic form of cell death. This publication shows that using glycosylated HBsAgS VLPs in the presence of aluminum hydroxide may result in a more immunogenic vaccine than that which is currently used. To study the effect of HBsAgS glycosylation, first N-terminal FLAG-tagged wild-type (WT) HBsAgS and point-mutated variants were expressed in HEK 293 cells. Variants used were threonine-to-asparagine mutant T116N and asparagine-to-glutamine mutant N146Q. The T116N mutant contained an additional asparagine available for glycosylation on the domain of HBsAgS which faces the lumen of the ER. On the other hand, the N146Q mutant lacked the asparagine which is typically N-glycosylated. SDS-PAGE followed by Coomassie staining revealed that about 50% of WT HBsAgS was glycosylated, running as two distinct bands at 27 kDa (glycosylated) 24 kDa (non-glycosylated). However, HBsAgS mutant T116N ran as two predominant bands at 27 kDa (monoglycosylated) and 29 kDa (diglycosylated). HBsAgS mutant N146Q ran as a single band at 24 kDa, indicating no glycosylation. This result confirmed that about half of HBsAgS produced in mammalian cells are N-glycosylated at N146 and no other amino acid. Both HBsAgS mutants formed VLPs similar to WT as viewed by transmission electron microscopy. VLPs were mostly spherical with some elongated in shape. Next, following removal of N-glycans using the enzyme peptide:N-glycosidase F (PNGase), quantitative N-glycome profiling was conducted using an advanced spectrometry technique called porous graphitized carbon liquid chromatography-electrospray ionization-tandem mass spectrometry (PGC-LC-ESIMS/MS). The T116N mutant was found to have a greater N-glycan density than WT HBsAgS, but a similar distribution of N-glycan types. Finally, the immunogenicity of glycoengineered HBsAg was tested using a mouse model of vaccination. BALB/c mice were immunized at weeks 1, 3, 5, and 7 with purified WT or T116N HBsAgS in the presence or absence of aluminum hydroxide. Some mice were immunized with Engerix-B as a control group. Serum samples were taken at weeks 2, 4, 6, 8, and 18 post-vaccination and analyzed by an ELISA assay against yeast-derived VLPs. Mice immunized with T116N HBsAgS combined with aluminum hydroxide had the highest titer of anti-HBsAgS antibodies at every time point tested. This indicates that hyper-glycosylated HBsAg is more effective than non-glycosylated HBsAg in mounting an immune response. The authors propose that hyper-glycosylated HBsAgS is more readily taken into antigen-presenting cells (APCs) due to an increased affinity for manose-binding lectin receptors expressed on those cells. Additionally, hyper-glycosylation of HBsAgS may lower its strength of adsorption with aluminum hydroxide, making it more prone to release and antigen processing. Taken together, these results demonstrate that glycoengineered HBsAgS formed VLPs and when combined with aluminum hydroxide, exhibited increased immunogenicity in BALB/c mice in comparison to a currently used vaccine. This publication shows one way in which molecular cloning techniques may be used to improve the efficiency and reliability of HBV vaccines.
- Caspase-6 Is a Key Regulator of Innate Immunity, Inflammasome Activation, and Host Defense – Cell
- This paper from St. Jude Children’s Research Hospital in Memphis, Tennessee shows that caspase-6 mediates inflammasome activation and plays a role in the activation of the programmed cell death (PCD) pathways pyroptosis, apoptosis, and necroptosis (PANoptosis). The caspase family of proteins are cysteine-aspartic proteases which cleave proteins between cysteine and aspartic acid residues. Caspases play essential rolls in inflammation and PCD pathways. Caspases exist as inactive zymogens (pro-forms) within the cell until they are cleaved, resulting their active form. Caspases are grouped as being either inflammatory (caspase-1, -4, -5, and -11) or apoptotic (caspase-3, -6, -7, -8, -9 and -10). However, emerging evidence has demonstrated crosstalk between these groups under certain conditions. Inflammatory caspases can play a role in PCD pathways and apoptotic caspases can play a role in inflammatory pathways. While caspase-6 has long been considered an executioner caspase in the apoptotic pathway, its major functions have remained unknown. This publication demonstrates that caspase-6 is an essential upstream component of Z-DNA binding protein 1 (ZBP1)-mediated inflammasome activation and subsequent PANoptosis. The NLR family pyrin domain-containing protein 3 (NLRP3) inflammasome is a multimeric structure consisting of NLRP3, apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC), and caspase 1 subunits. NLRP3 inflammasome activation results in caspase-1 mediated cleavage of pro-interleukin 1β (IL-1β) as well as gasdermin D into their active forms. Cleaved gasdermin D forms pores in the cell membrane resulting in the rapid release of pro-inflammatory IL-1β and ultimately causing pyroptosis. The NLRP3 inflammasome can be activated by a variety of stimuli including canonical stimuli (pore-forming toxins, ATP) and non-canonical stimuli (intracellular LPS sensed by caspase-4/5). Additionally, this group has previously demonstrated that the NLRP3 inflammasome can also be activated by ZBP1 sensing of influenza A virus (IAV). In order to discern if caspase-6 is involved in NLRP3 inflammasome activation, bone marrow-derived macrophages (BMDMs) were derived from caspase-6 knockout (Casp6–/–) mice. Caspase-6 was shown to be dispensable for both canonical and non-canonical activation of the NLRP3 inflammasome, as caspase-1 cleavage was shown via Western blot and secretion of both IL-1β and IL-18 was shown via ELISA. However, when infected with IAV, Casp6–/– BMDMs failed to display caspase-1 cleavage and cytokine release compared to the wild-type (WT) control. This indicates that caspase-6 plays an essential role in IAV-induced NLRP3 inflammasome activation and pyroptosis. As this group and others have shown that ZBP1 regulates various forms PCD in response to IAV infection, next the roll of caspase-6 in PCD pathways was investigated. Overall cell death 12 hours following IAV infection was reduced by about 50% in Casp6–/– BMDMs as measured by SYTOX Green nucleic acid stain and high-content imaging. To investigate this phenomenon further, CRISPR-Cas9 was used to generate caspase-6 knockout (Casp6KO) mouse embryonic fibroblasts (MEFs). IAV-induced cell death was largely ablated in Casp6KO MEFs compared to WT MEFs as measured by SYTOX Green nucleic acid stain and high-content imaging. Furthermore, Casp6KO MEFs showed highly reduced IAV-induced cleavage of apoptotic caspases-3, -7, and -8 as measured by Western blot. Additionally, Casp6–/– BMDMs showed highly reduced cleavage of the pyroptosis effector gasdermin D and phosphorylation of the necroptosis effector pseudokinase mixed lineage kinase domain-like (MLKL) upon IAV infection. Taken together, these results indicate that caspase-6 plays a critical role in the IAV-induced PCD pathways pyroptosis, apoptosis, and necroptosis. Interestingly, Casp6–/– BMDMs were still susceptible to necroptosis by the classical trigger of TNFα plus zVAD, indicating an IAV-specific necroptotic function of caspase-6. In a mouse model, the authors found that caspase-6 deficiency increased susceptibility to IAV infection. Upon IAV infection, ZBP1 recruits RIPK1 and RIPK3 via the receptor-interacting protein homotypic interaction motif (RHIM) to form a cell death complex. It has been demonstrated that from this complex, RIPK3 activates parallel pathways of apoptosis and necroptosis. In order to explore if this complex directly regulates caspase-6 cleavage, Ripk3–/– and Zbp1–/– BMDMs were utilized. Both Ripk3–/– and Zbp1–/– BMDMs showed reduced cleavage of caspase-6, -8, -7, -3 and gasdermin D as well as reduced MLKL phosphorylation. This result confirms the previous finding that in response to IAV infection, ZBP1 and RIPK3 mediate both apoptotic and necroptotic pathways and suggests a third role for RIPK3 in IAV-induced, ZBP1-mediated pyroptosis. This result also indicates that caspase-6 is regulated at the level of the ZBP1-RIPK3 complex when taken together with the finding that caspase-6 deletion affected all three forms of PCD. Additionally, similar experiments using BMDMs lacking either gasdermin D or NLRP3 both showed no change in caspase-6 cleavage. To determine which protein in the ZBP1-RIPK3 complex interacts with caspase-6, components of the complex (RIPK1, RIPK3, ZBP1, caspase-8) were individually over-expressed in HEK293T cells via transfection alongside a catalytically dead, FLAG-tagged caspase-6, followed by co-immunoprecipitation (Co-IP) using an anti-FLAG antibody. Only RIPK3 was pulled down alongside FLAG-caspase-6, indicating that caspase-6 interacts with RIPK3. Further Co-IP experiments in immortalized BMDMs utilizing a doxycycline-inducible FLAG-caspase-6 showed that increased levels of caspase-6 improved the ability of RIPK3 to interact with ZBP1. This indicates that caspase-6 may promote IAV-induced PANoptosis by facilitating the interaction of ZBP1 with RIPK3. This paper identifies a previously unknown role for caspase-6 in regulating ZBP1-mediated inflammasome activation and PANoptosis. Additionally, caspase-6 was shown to be essential for host defense against AIV in a mouse model. The results presented here further elucidate the complex interactions of cell death effectors in the context of IAV infection. These findings may help in the development of novel IAV therapies as well as treatments for diseases with abnormally regulated cell death pathways.
Meet our guest blogger, David Schad, B.Sc., Junior Research Fellow at the Baruch S. Blumberg Institute studying programmed cell death such as 



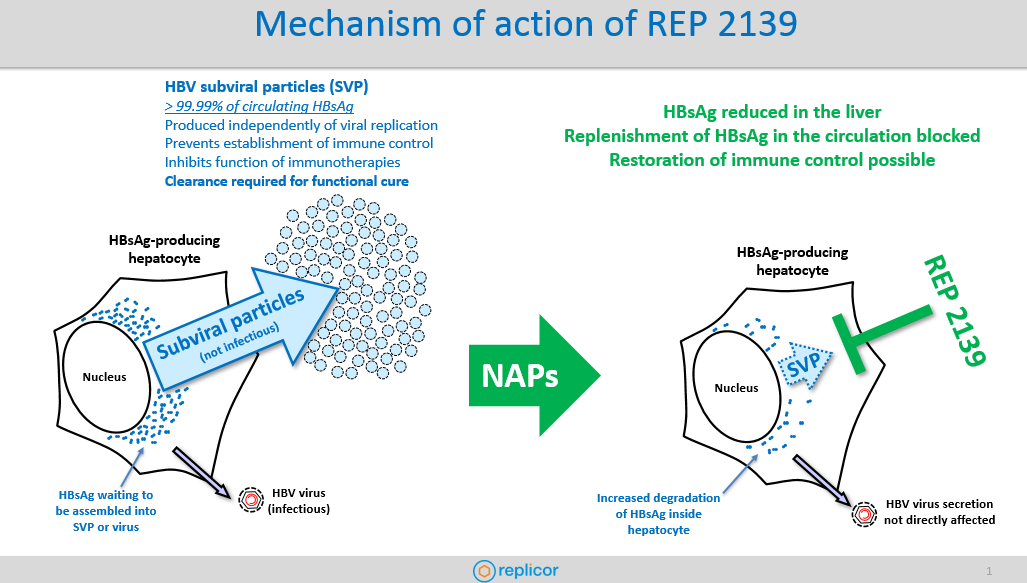
 speak with Andrew Vaillant, Ph.D., Chief Scientific Officer at Replicor at the annual International HBV Meeting in Melbourne, Australia. Dr. Vaillant gave us an inside look at REP 2139 – their drug candidate developed for the treatment of chronic hepatitis B and HBV/HDV coinfection. REP 2139 is a nucleic acid polymer that removes surface antigen (HBsAg) and as part of combination therapy, has achieved functional cure for chronic HBV (sustained HBsAg loss) and sustained clearance of HDV infection from the blood in early phase II proof of concept clinical trials it has completed to date.
speak with Andrew Vaillant, Ph.D., Chief Scientific Officer at Replicor at the annual International HBV Meeting in Melbourne, Australia. Dr. Vaillant gave us an inside look at REP 2139 – their drug candidate developed for the treatment of chronic hepatitis B and HBV/HDV coinfection. REP 2139 is a nucleic acid polymer that removes surface antigen (HBsAg) and as part of combination therapy, has achieved functional cure for chronic HBV (sustained HBsAg loss) and sustained clearance of HDV infection from the blood in early phase II proof of concept clinical trials it has completed to date.