
Although there are highly effective treatments available to manage hepatitis B, there are few available treatments for hepatitis D, and none are U.S. Food and Drug Administration (FDA) approved. Hepatitis D is the most severe form of viral hepatitis, and coinfection can accelerate liver damage and cause cirrhosis or liver cancer in as little as 5 years for some patients. Currently there is no approved drug for acute or chronic hepatitis B/D coinfection, but in trials pegylated interferon alpha has shown to be somewhat effective. By stimulating the body’s immune system, around 25-30% of patients are able to suppress their hepatitis D viral load with weekly injections over 48 weeks. Emerging research is showing higher rates of effectiveness with prolonged interferon treatment beyond one year, but it can be difficult for patients to continue due to the physical and mental toll of interferon on the body. Antiviral medications that are proven effective against hepatitis B are sometimes prescribed along with interferon therapy for patients with a high hepatitis B viral load, but these have no effect on hepatitis D. It is urgent that more treatment options be developed for the millions of hepatitis B/D patients that are eagerly awaiting them.
The good news is that with renewed scientific interest, research and funding, eight new drugs are currently in development that offer hope for more treatment options in the coming years. Two drugs have even been granted special designations by the FDA and one by European Medicines Agency (EMA), paving the way for increased resources and funding for development. Due to recent advancements, the future looks hopeful, and within a few years it is likely there will be more treatment options available. Below is a chart that provides more information on these new drugs and their current clinical trial status.
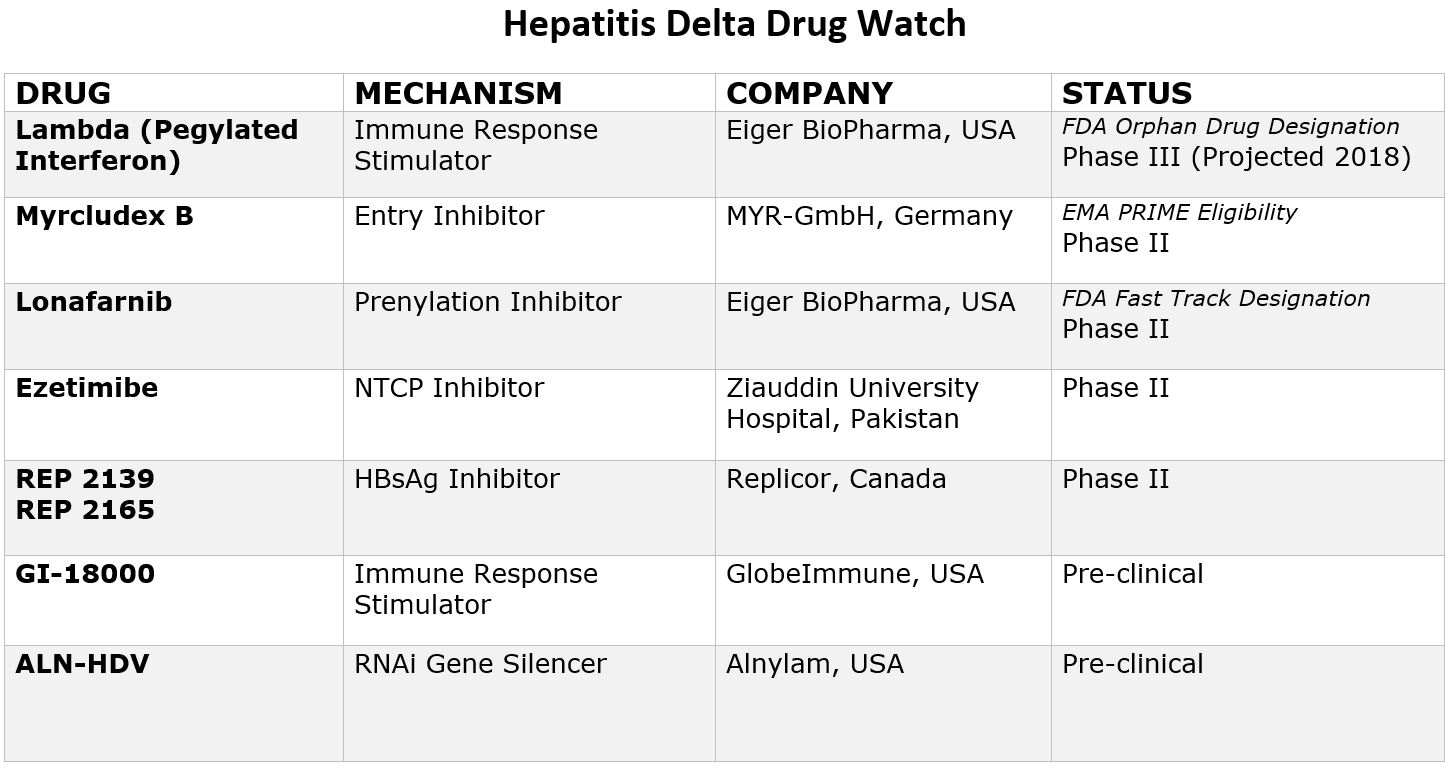
Pegylated Interferon Lambda
Pegylated-interferon-lambda (PEG-IFN-λ) is a well-characterized, late-stage, first in class, type III interferon that stimulates cell-mediated immune responses that are critical for the development of host protection during viral infections. This drug has now been granted “Orphan Drug Designation” by the FDA, fast-tracking the development process.
Myrcludex B
This drug is an “entry inhibitor” that prevents the virus from entering into hepatocytes (liver cells) and has shown activity against the hepatitis B virus. It may also stop the development of a hepatitis D infection. A recent study showed promise for Myrcludex B when combined with PEG-INF in reducing hepatitis D viral levels. It has been granted PRIME Eligibility by the European Medicines Agency, a status that promotes support in development of drugs that serve an unmet medical need.
Ezetimibe
Currently used to lower cholesterol in the blood, Ezetimibe is being studied for effectiveness against hepatitis D. Ezetimibe possesses pharmacophore features to stop NTCP, the receptor required for hepatitis B and hepatitis D hepatocyte entry.
Lonafarnib
This drug works by targeting the protein assembly process, preventing the production of new virus particles. In a current clinical trial, Lonafarnib combined with Ritonavir has shown promise in reducing hepatitis D viral levels, and the FDA has granted it fast-track status since this class of drugs have been developed for the treatment of cancers and have been shown to be safe.
Rep 2139
This compound is known as a “Nucleic acid-based Amphipathic Polymer” (NAP) which prevents the release of hepatitis B surface antigen (HBsAg) from infected liver cells and is being evaluated for hepatitis D virus in combination with pegylated interferon (PEG IFN).
GI-18000
GI-18000 Tarmogen is being studied for its effectiveness in causing a T cell immune response against cells infected with Hepatitis D and thereby improving outcomes. The strategy is to identify molecular targets that distinguish diseased cells from normal cells and activate the immune system to selectively target and eliminate only the diseased cells.
ALN-HDV
This approach is being used for both the hepatitis B and hepatitis D virus to “silence” the viral RNA with compounds that interfere with and cause the destruction of the viral genome (e.g. stop replication of the virus).
As clinical trials progress, sites may open across the world that are enrolling hepatitis D patients. Keep checking here for an up-to-date list of all current clinical trials.
Click here for more information about the phases of the clinical trial process.
For more information about hepatitis B/D coinfection, please visit www.hepdconnect.org or email us at connect@hepdconnect.org.

